For decades, cancer treatment meant one thing: poison the fast-growing cells and hope the good ones survive. Chemotherapy wiped out tumors-but also hair, gut lining, and energy. Then came something different. Not just stronger drugs, but smarter ones. Targeted therapy doesn’t guess. It reads the DNA of your tumor and attacks only what’s broken. This isn’t science fiction. It’s happening right now, in hospitals from Manchester to Memphis, changing how people live with cancer.
What Targeted Therapy Actually Does
Targeted therapy is cancer treatment based on the specific genetic mistakes inside a tumor. Think of it like finding the exact lock that’s jammed in a door, then using one key to open it-not a crowbar. These drugs block signals that tell cancer cells to grow, divide, and spread. They don’t touch healthy cells the way chemo does. That’s why side effects are often lighter: no constant nausea, fewer infections, less fatigue.
The first big win came in 2001 with imatinib (Gleevec) for chronic myeloid leukemia. Before that, most patients died within a year. With imatinib, 89% were alive after 12 months. That wasn’t just an improvement-it was a revolution. Today, 73% of all new cancer drugs approved by the FDA are targeted therapies. They’re not just options anymore. For some cancers, they’re the standard.
How Doctors Find the Right Target
Before giving a targeted drug, doctors need to know what they’re targeting. That means testing the tumor’s DNA. This isn’t a simple blood test. It’s next-generation sequencing (NGS), which reads hundreds of genes at once. Panels like FoundationOne CDx or MSK-IMPACT scan 300 to 500 cancer-related genes. You need just 20-50 nanograms of tumor DNA-about the weight of a single grain of salt-to get results.
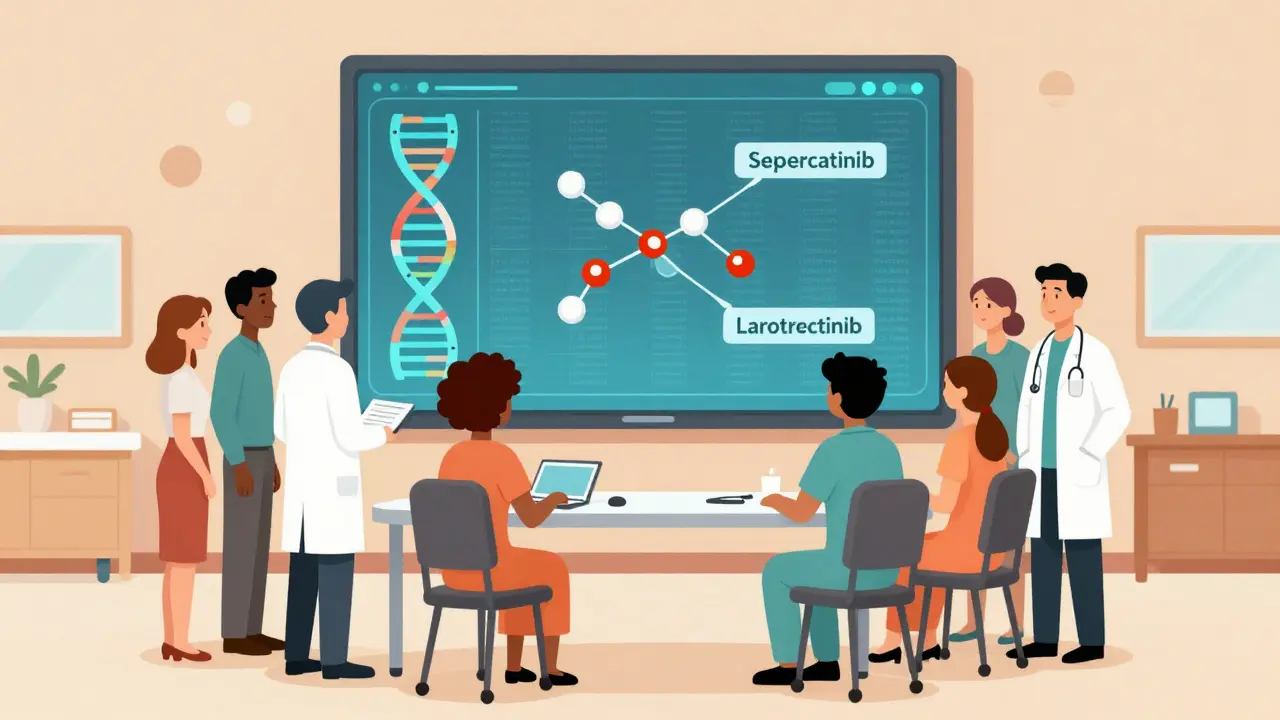
But it’s not just about finding mutations. It’s about knowing which ones matter. Some changes are harmless noise. Others are the engine of the cancer. The big targets? Mutations in genes like EGFR, ALK, BRAF, and ROS1 in lung cancer. HER2 in breast cancer. NTRK fusions in rare tumors. RET mutations in thyroid and lung cancers. Each has a matching drug. Selpercatinib, for example, shrinks tumors in 85% of lung cancer patients with RET mutations-compared to 30-40% with chemo.
And here’s the wild part: some drugs work across cancer types. Larotrectinib treats any tumor with an NTRK fusion-whether it’s in the lung, colon, or salivary gland. That’s called histology-agnostic therapy. The tumor’s origin doesn’t matter. Only its genes do.
Why It Works Better-For Some
Targeted therapy isn’t magic. It only works if your tumor has the right mutation. In EGFR-mutant non-small cell lung cancer, osimertinib extends survival without progression to nearly 19 months-almost double the 10 months you get with chemo. But if you don’t have that mutation? The drug does almost nothing. Response rates drop to 2-5% in unselected patients.
That’s why testing is non-negotiable. Without it, you’re gambling. And the cost of that gamble? Time, side effects, and hope. But when it works, the difference is stark. A patient with stage IV lung cancer on osimertinib often reports shrinking tumors in weeks, no hair loss, and the ability to keep working. One woman on CancerCare’s forum said, “I went from needing help to walk to the kitchen to hiking with my grandchildren in six months.”

The Hard Truths
Not everyone benefits. Only about 13.8% of cancer patients have a tumor with a currently actionable genetic target. That number is growing, but slowly. And even when you qualify, resistance almost always shows up. In 70-90% of cases, the cancer finds a way around the drug within 9 to 14 months. It’s like the tumor learns to wear a new lock.
Another problem? Access. In the U.S., 65% of advanced cancer patients get genomic testing. In Europe, it’s 22%. In Asia, just 8%. Insurance often denies coverage for testing-55% of patients in one survey reported delays or denials. Some wait over a month just to get a test approved. And when they finally get it? The drug might cost $15,000 to $30,000 a month. Chemo? $5,000 to $10,000. Forty percent of patients on targeted therapy report financial hardship.
Then there’s the “molecular frustration” problem. A patient with a rare NTRK fusion might qualify for larotrectinib, but their insurance says, “It’s not standard for colon cancer.” Even though the drug works across all cancers with that mutation. The system hasn’t caught up to the science.
Who Gets Left Behind?
Targeted therapy isn’t just expensive-it’s unequal. Black and Latino patients are less likely to get tested. Rural patients often can’t reach a center with NGS capabilities. Community oncologists, who treat most cancer patients, rarely have access to molecular tumor boards. Only 32% of community hospitals have them. Meanwhile, NCI-designated centers have them in 89% of cases.
And even when you get tested, results can be confusing. About 20-30% of gene reports show “variants of unknown significance.” That means: we found a change, but we don’t know if it’s driving the cancer or just a harmless glitch. No drug exists for that. No clear path forward. That’s where genetic counselors become essential. The best programs have one counselor for every 150 patients.
What’s Next?
The future is already here. Liquid biopsies-blood tests that catch tumor DNA floating in the bloodstream-are now FDA-approved. Guardant360 can detect resistance mutations months before a scan shows growth. That means doctors can switch drugs before the cancer spreads.
Artificial intelligence is helping too. IBM Watson for Oncology matches tumor profiles to treatment options with 93% accuracy compared to expert tumor boards. AI won’t replace doctors-but it’s making precision medicine faster and more consistent.
And researchers are chasing the next big hurdle: tumor suppressor genes. These are the brakes that cancer disables. TP53, PTEN, BRCA-they’re mutated in 80% of cancers. But no drug can restore a broken brake. That’s the next frontier. The NCI’s $195 million RESPOND initiative is trying to fix the racial gaps in access. Project Orbis is speeding up global approvals. And combination therapies-hitting cancer with two or three targeted drugs at once-are showing early promise in trials.
What This Means for You
If you or someone you love has advanced cancer, ask: “Has my tumor been genetically tested?” Don’t assume it has. Push for it. If your doctor says, “We don’t do that here,” ask for a referral to a comprehensive cancer center. Organizations like the Personalized Oncology Alliance offer free molecular tumor board reviews for community practices.
Insurance denials are common. If your test or drug is denied, appeal. Document everything. Patient advocacy groups like the American Cancer Society and Cancer Support Community have teams that help with appeals and financial aid.
Targeted therapy isn’t a cure-all. But for those who qualify, it turns a death sentence into a manageable condition. It’s not perfect. It’s not fair. But it’s real. And it’s changing lives-one gene at a time.
How is targeted therapy different from chemotherapy?
Chemotherapy attacks all fast-growing cells-cancerous and healthy-leading to side effects like hair loss, nausea, and low blood counts. Targeted therapy only attacks cancer cells with specific genetic changes. It’s like using a scalpel instead of a sledgehammer. Side effects are usually milder, though they can still include rashes, diarrhea, or high blood pressure depending on the drug.
Can targeted therapy cure cancer?
For most cancers, targeted therapy doesn’t cure-but it can control the disease for years. In some cases, like chronic myeloid leukemia with imatinib, patients live nearly normal lifespans. For others, like advanced lung cancer with EGFR mutations, survival has doubled or tripled. The goal is long-term management, not always complete elimination.
How long does genetic testing take?
Most comprehensive tumor DNA tests take 14 to 21 days from the time the sample is sent to the lab. Some faster tests, like those for EGFR or ALK, can return in 7-10 days. But delays happen-insurance approvals, sample quality issues, or lab backlogs can push it to 4-6 weeks. Always ask your care team for a timeline.
What if my tumor has no known target?
About 85% of solid tumors don’t have an approved targeted therapy yet. That doesn’t mean there’s no hope. You may still benefit from immunotherapy, clinical trials, or combination treatments. Ask your oncologist about molecular tumor boards-they review complex cases and sometimes find off-label options or trial matches you didn’t know existed.
Are targeted therapies covered by insurance?
Most FDA-approved targeted therapies are covered, but only if you meet strict criteria-like having the right mutation confirmed by an approved test. Testing itself is often denied or delayed. If you’re denied, file an appeal with your insurer and ask your doctor to write a letter of medical necessity. Patient advocacy groups can help you navigate this process.
Can I get targeted therapy without a biopsy?
Sometimes. Liquid biopsies-blood tests that detect tumor DNA-can be used when a tissue biopsy is too risky or not possible. They’re FDA-approved for certain cancers, like lung cancer with EGFR mutations. But they’re not as reliable as tissue tests for all mutations. A tissue biopsy is still the gold standard when feasible.

rajaneesh s rajan
January 30, 2026 AT 04:33So we’re telling cancer to play chess while we’re still using a sledgehammer? Honestly, it’s wild how long we waited to start reading the rulebook instead of just smashing the board. I’ve seen friends go from chemo hell to hiking with grandkids on these drugs - no wig needed, no vomiting in the bathroom at 3am. But then you remember: only 1 in 7 get to play this game. The rest? Still stuck with the sledgehammer.
Frank Declemij
January 31, 2026 AT 03:37Targeted therapy isn’t magic. It’s biology. The fact that we can now sequence a tumor’s genome and match it to a drug that specifically inhibits a mutated kinase is a triumph of molecular oncology. The real failure isn’t the science - it’s the infrastructure. Insurance delays, lack of access in rural areas, and the myth that all cancers are the same. We need systemic change, not just better drugs.
Sheryl Dhlamini
February 1, 2026 AT 12:04I lost my mom to lung cancer in 2018. They never tested her tumor. Said it was ‘too late.’ Six months later, they approved a test for her type - and there was a drug. A good one. I scream into the void sometimes about this. It’s not just about science. It’s about who gets to live. And who gets told ‘we don’t do that here.’
Doug Gray
February 1, 2026 AT 14:21Okay but like… what if the tumor just… doesn’t wanna be targeted? 😅 Like I get the science, but it feels like we’re putting all our eggs in this one basket and pretending it’s not gonna evolve. Also, the price tag? Bro. I’ve seen people sell their cars for a month of this stuff. And then the insurance says ‘not medically necessary’ because the mutation is ‘rare for colon cancer’ even though the drug works on ALL NTRK fusions. The system is broken. Not the science.
LOUIS YOUANES
February 2, 2026 AT 17:50Let’s be real - this is Big Pharma’s new golden goose. They didn’t invent targeted therapy to save lives. They invented it because they realized they could charge $25K/month for a pill that only works on 13% of people. And now they’ve got the whole medical establishment kissing their ass because ‘precision medicine’ sounds so damn sexy. Meanwhile, chemo’s still cheaper, more widely available, and actually works on more people. Wake up.
Pawan Kumar
February 3, 2026 AT 23:52Genetic testing? In India? Ha. Only the rich get to play. My cousin got diagnosed with stage IV gastric cancer - they sent the sample to the US for sequencing. Took 9 weeks. By then, he was too weak to even swallow the drug. Meanwhile, the hospital charged him 8 lakhs for ‘consultation’. The system is designed to fail the poor. This isn’t medicine. It’s a luxury subscription.