Tag: standard review FDA
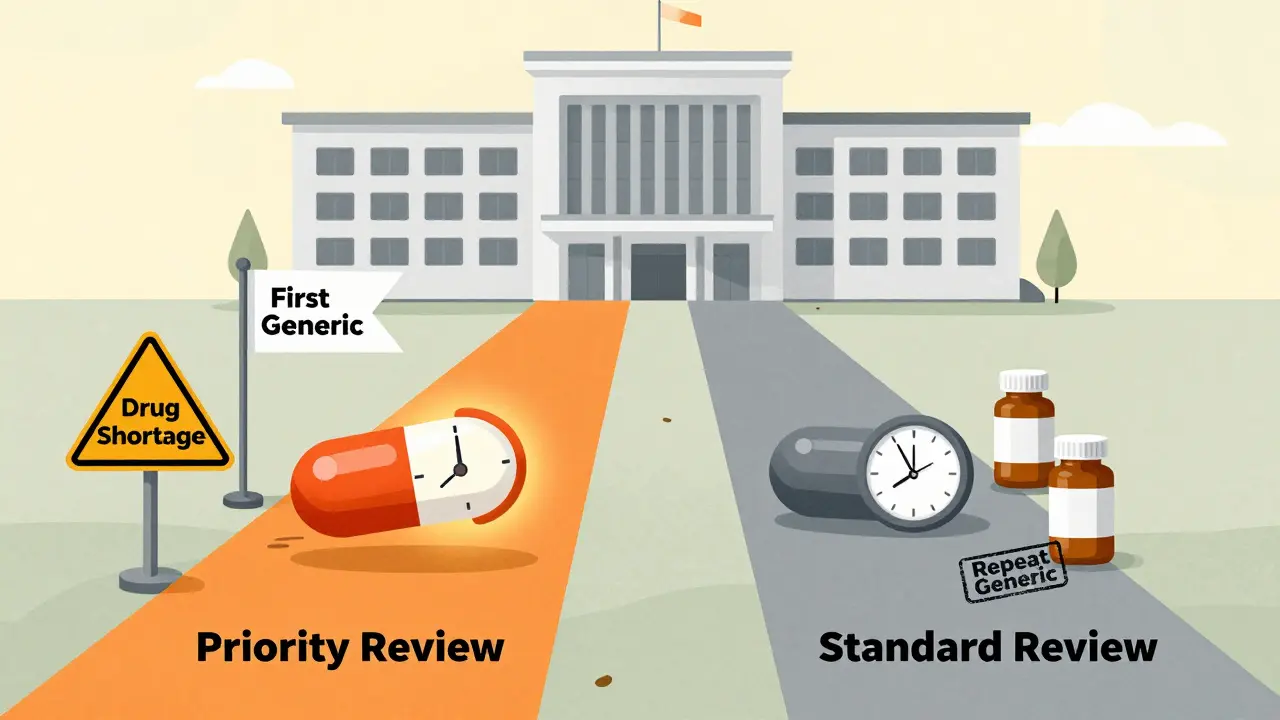
Priority vs Standard Review: How FDA Prioritizes Generic Drug Applications
The FDA uses priority and standard review paths for generic drugs. Priority review speeds up approval by two months for first generics, drugs in shortage, or those made in the U.S. This impacts pricing, supply, and patient access.
